Introduction
Sepsis is a critical medical emergency caused by an uncontrolled immune response to infection, resulting in organ dysfunction. It continues to be a major global health challenge with high morbidity and mortality. During sepsis, inflammation becomes excessive, triggering widespread physiological changes that disrupt normal immunity and blood flow.
A key component of this process is immunothrombosis, where the immune and coagulation systems interact. While this mechanism normally helps trap pathogens, its dysregulation in sepsis leads to harmful microvascular clots, tissue ischemia, and severe complications such as disseminated intravascular coagulation (DIC). Understanding the cellular mediators and signaling pathways involved is essential to developing effective therapeutic strategies.
Pathophysiology of Sepsis-Induced Immunothrombosis
Immunothrombosis is a natural defense process where the immune and clotting systems work together to contain pathogens. However, in severe infection or critical illness like sepsis, this controlled response becomes excessive, leading to the formation of harmful blood clots and organ injury.
Sepsis-Induced Hyperinflammation
In sepsis, the immune system overreacts to pathogens, releasing large amounts of cytokines and inflammatory mediators. This uncontrolled inflammation, often called a cytokine storm, damages tissues, disrupts blood vessels, and activates coagulation. Persistent exposure to PAMPs and DAMPs keeps immune cells activated, worsening inflammation and driving clot formation.
Sepsis-Induced Immunothrombosis
Excessive inflammation disrupts the balance between clot formation and clot breakdown, leading to widespread thrombosis, often progressing to DIC. Elevated fibrinogen and excessive fibrin formation contribute to a hypercoagulable state, while impaired fibrinolysis prevents clot resolution. This imbalance results in sepsis-induced coagulopathy and microvascular obstruction.
Crosstalk Between Inflammation and Coagulation
Inflammation and coagulation reinforce each other in sepsis. Inflammatory cytokines stimulate tissue factor expression and thrombin generation while suppressing natural anticoagulant pathways. Endothelial injury and glycocalyx damage further promote immune cell adhesion and clot formation, driving microthrombi development and worsening tissue damage.
Cellular Activation
Cellular Activation in Sepsis-Induced Immunothrombosis
Innate immune cells and endothelial cells play a central role in the development of immunothrombosis during sepsis. Myeloid cells—particularly monocytes, macrophages, and neutrophils—contribute through tissue factor (TF) expression, NET formation, and abnormal signaling pathways. Platelets and endothelial cells further support microthrombus formation in severe sepsis. (Figure 2 illustrates key cellular contributors and mechanisms.)
Activation of Leukocytes
Leukocytes detect and respond to pathogens through receptors such as TLRs and cytokine receptors. During sepsis, these cells undergo functional and structural changes, contributing to inflammation and coagulation. Despite activation, leukocyte numbers may decrease over time, resulting in leukopenia and immune suppression.
Activation of Monocytes
Monocytes and macrophages initiate inflammation through phagocytosis and cytokine release. In sepsis, TLR activation by PAMPs and DAMPs induces tissue factor release, triggering coagulation and promoting thrombosis, as shown in Figure 1. Monocytes also release TF-rich extracellular vesicles and differentiate into proinflammatory M1 macrophages, further amplifying cytokine production and interacting with platelets and endothelial cells.
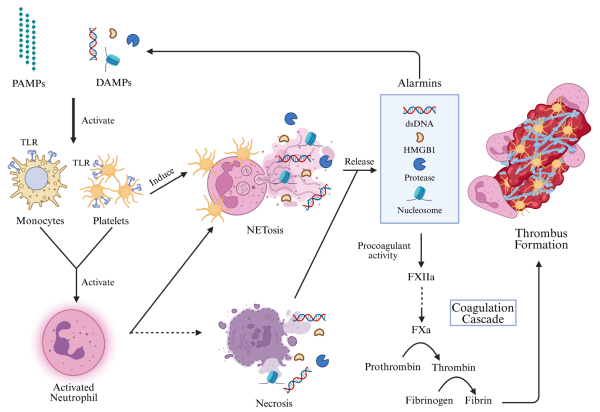
Figure 1 – Immunothrombosis and thromboinflammation in septic infection.
Activation of Neutrophils
Neutrophils in Immunothrombosis
Neutrophils are key early responders during infection and become fully active after maturation, guided by receptors like CXCR2 and TLRs. Beyond pathogen killing, they play an important role in coagulation during sepsis. (Figure 2 shows neutrophil involvement in clot formation.)
NET Formation and Thromboinflammation
Neutrophils form extracellular traps (NETs)—web-like DNA structures containing enzymes such as elastase and MPO—that help capture pathogens. However, in sepsis, NETs also activate coagulation factors, suppress anticoagulant pathways, and promote endothelial dysfunction, contributing to pathological clot formation.
Activation of Platelets
Platelets not only support clotting but also act as immune regulators through receptors like TLRs and complement receptors. During sepsis, they become hyperactivated, releasing mediators like ADP and thromboxane A2, promoting aggregation and excessive clotting.
Platelet Interactions in Sepsis
Activated platelets form complexes with monocytes and neutrophils through molecules like P-selectin, amplifying inflammation and NET formation (shown in Figure 2). These interactions further enhance thrombin generation, contributing to immunothrombosis, thrombocytopenia, and a higher risk of organ failure.
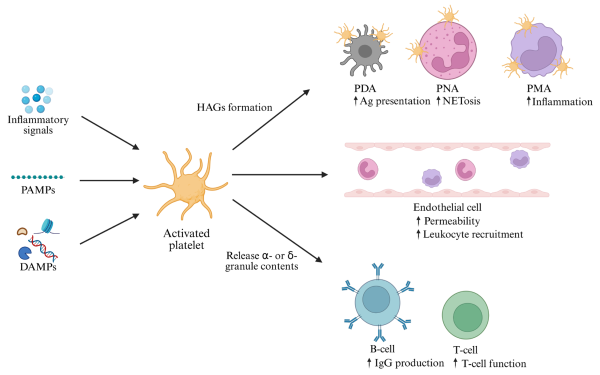
Figure 2 – Activation of neutrophils and formation of NETs.
Activation of ECs
Endothelial cells normally maintain anti-inflammatory and anticoagulant balance, but in sepsis they are rapidly activated by PAMPs, cytokines, and NETs. Through TLR signaling, ECs increase the expression of adhesion molecules such as ICAM-1, VCAM-1, E-selectin, and VWF, supporting leukocyte and platelet attachment.
Damage to the endothelial glycocalyx increases vascular permeability, edema, and promotes cellular aggregation. Activated ECs also release large VWF multimers and express tissue factor, driving excessive platelet adhesion and microthrombus formation.
Cellular Signaling Pathways in Immunothrombosis
Overview of Dysregulated Signaling in Sepsis
Sepsis begins when immune cells detect PAMPs or DAMPs through TLRs, triggering excessive inflammation and coagulation. This unregulated response activates pathways such as NF-κB, MAPK, JAK-STAT, NLRP3 inflammasomes, and cGAS-STING, driving SIRS, organ dysfunction, and prothrombotic conditions.
NLRP3 Inflammasome Pathway
The NLRP3 inflammasome rapidly responds to infection by activating caspase-1 and releasing IL-1β and IL-18. Its activation promotes pyroptosis and tissue factor (TF) release, initiating the coagulation cascade and thrombin generation. This pathway is further amplified by cGAS-STING and NF-κB signaling.
cGAS-STING Pathway
cGAS-STING is triggered by abnormal or circulating DNA, especially mitochondrial DNA in sepsis. This pathway activates gasdermin D–mediated pyroptosis, increases TF release, and enhances inflammatory signaling through the STING–IRF3–NF-κB axis, contributing to coagulation dysregulation.
Other Signaling Pathways
The PI3K/Akt pathway regulates platelet activation, integrin signaling, and thrombus stabilization. Neutrophils reinforce thrombosis by forming NETs, which activate coagulation factors, degrade anticoagulants, and interact with ECs and platelets. Together, immune cells—especially monocytes, neutrophils, and platelets—drive inflammasome activation, cytokine release, and microthrombus formation during sepsis.
Molecular Mechanisms of Sepsis-Induced Immunothrombosis
Immunothrombosis Overview
Immunothrombosis links inflammation and coagulation. While it helps control pathogens, its dysregulation leads to pathological clotting, endothelial injury, NETosis, and higher mortality. Platelets, leukocytes, and endothelial cells act as key drivers of this process.
Role of Non-Coding RNAs
Non-coding RNAs—especially miRNAs—regulate immune responses, TLR signaling, and inflammatory balance. Dysregulated miRNAs like miR-146a, miR-223, miR-122, and miR-150 are associated with sepsis severity, NETosis, and thrombosis.
lncRNAs, circRNAs & Pathway Modulation
lncRNAs and circRNAs influence pyroptosis, inflammasome activation, TF expression, and metabolic pathways such as PI3K/Akt. Their dysregulation supports endothelial dysfunction, platelet activation, and immunothrombotic progression.
Exosomes, mtDNA & DAMP Signaling
Extracellular vesicles, histones, and cell-free mitochondrial DNA amplify cytokine release, immune activation, and TF expression via NF-κB, MAPK, and TLR pathways, further driving coagulation in sepsis.
miRNAs as Biomarkers
Multiple miRNAs linked to cytokines, TF, fibrinogen, and coagulation factors serve as potential diagnostic and prognostic biomarkers. miRNA-223a, in particular, shows strong diagnostic promise for sepsis.
Genomic profiling, platelet transcriptomics, and deep learning-based biomarkers are improving early detection and monitoring of sepsis-induced coagulopathy. Understanding cell-specific regulation may guide targeted therapeutic strategies.
Therapeutic Strategies for Sepsis-Induced Immunothrombosis
Overview of Treatment Challenges
Sepsis-induced immunothrombosis is complex and difficult to treat, with no single effective therapy. Current treatment combines antibiotics with anticoagulants, but long-term use increases bleeding risk.
Anticoagulant-Based Therapies
Heparin is the most widely used agent due to its anticoagulant and anti-inflammatory effects. Other natural anticoagulants like APC, antithrombin, TFPI, and thrombomodulin have also been tested, but bleeding remains a major limitation.
Anti-Platelet and Targeted Coagulation Therapies
Drugs like clopidogrel, ticagrelor, and GP IIb/IIIa inhibitors help reduce platelet activation and thrombosis. Newer therapies targeting tissue factor pathways and NET formation (e.g., PADI4 and CXCR1/2 inhibitors) show promising results.
Immune-Modulating and Anti-inflammatory Approaches
Targeted approaches including CD14 antibodies, inflammasome inhibitors like MCC950, and molecules such as Emelin or amitriptyline help reduce cytokine storm, inflammation, and platelet activation. Histidine-rich glycoprotein also prevents immunothrombus formation.
Despite many studies, most therapies have shown limited mortality benefit and safety concerns. However, combination treatments appear more promising, highlighting the need for multimodal and personalized strategies.
Conclusion
Immunothrombosis is a natural defense mechanism where inflammation and coagulation work together to trap and contain pathogens. However, in sepsis this process becomes uncontrolled, leading to pathological clotting, inflammation, and complications like thromboinflammation and DIC. Immune cells, platelets, and endothelial dysfunction drive excessive coagulation, supported by key signaling pathways such as NLRP3, NF-κB, and JAK-STAT. Non-coding RNAs, especially miRNAs, also regulate these pathways, and their dysregulation worsens inflammation and thrombosis. While anti-inflammatory and anticoagulant therapies help manage sepsis-induced coagulopathy, many carry bleeding risks. Therefore, safer and more targeted therapeutic strategies are needed for better clinical outcomes.
Source: Aklilu, A.; Lai, M.S.-L.; Jiang, Z.; Yip, S.P.; Huang, C.-L. Immunothrombosis in Sepsis: Cellular Crosstalk, Molecular Triggers, and Therapeutic Opportunities—A Review. Int. J. Mol. Sci. 2025, 26, 6114.
https://doi.org/10.3390/ijms26136114