Introduction
Invasive candidiasis is a serious fungal infection seen in 2–10% of ICU patients, driven by Candida species that can enter the bloodstream, form biofilms, and spread to multiple organs. Diagnosis is difficult, blood cultures are slow and often insensitive, while molecular tests and β-D-glucan provide quicker results but lack consistency and specificity. Delayed treatment significantly increases mortality, making early detection and timely antifungal therapy essential.
Risk rises with immunosuppression, invasive devices, major surgery, broad-spectrum antibiotics, and prolonged ICU stay. Preventive measures such as strict hygiene, antifungal stewardship, and routine screening for resistant species like C. auris are crucial. Emerging biomarkers, including siderophores, may enhance early diagnosis in the future, supporting better clinical outcomes and reducing complications.
Diagnosis of Invasive Candidiasis
The diagnosis of IC represents a significant medical challenge, particularly in patients with sepsis, systemic inflammatory response syndrome, or severe immunosuppression, where rapid and accurate recognition of the infection is of paramount importance. The limitations of traditional laboratory diagnostic methods have led to the development of advanced techniques and new biomarkers for faster, more reliable detection. Delayed diagnosis and treatment are associated with an increased mortality rate and the importance of early diagnosis and treatment initiation cannot be overstated. Figure 1 illustrates the diagnostic algorithm for using the various laboratory techniques in patients at risk of invasive candidiasis.
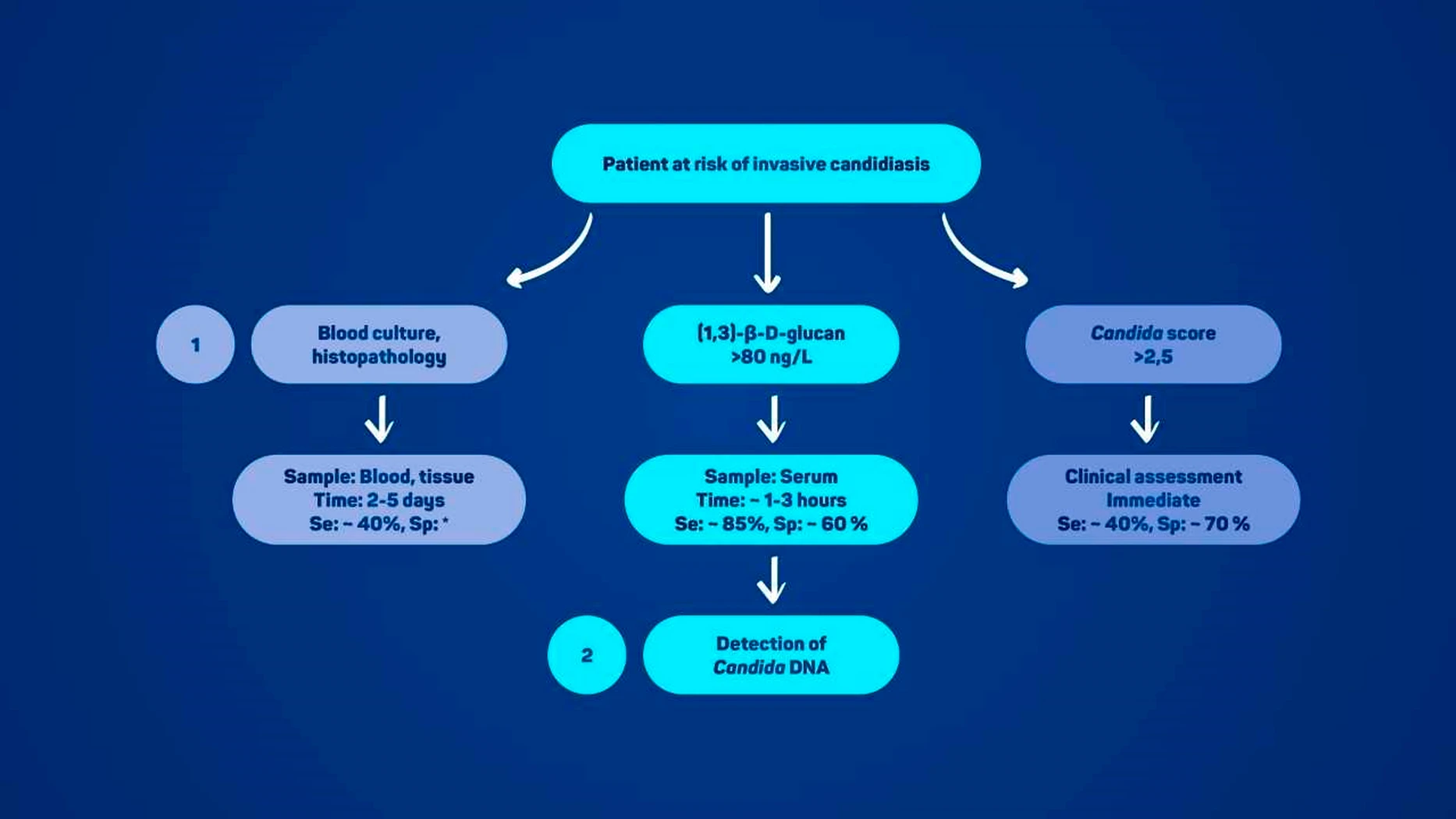
Figure 1
Proof of Infection
Gold Standard Methods for Diagnosing Invasive Candidiasis
Blood culture is the gold standard for diagnosing candidemia, but it has low sensitivity (50–75%) and takes 2–5 days because yeast levels in blood are very low and need time to multiply. Prior antifungal use can also cause false negatives. Histopathology is highly specific but invasive, requires tissue biopsy, and has low sensitivity due to uneven yeast distribution, making it unsuitable for patients with bleeding risks.
Newer methods such as PCR-based tests, T2Candida® (previously used), and biomarkers like β-D-glucan provide faster, more sensitive, and less invasive detection, even when antifungals have already started. However, they can give false positives, cannot determine drug susceptibility, and may be costly. Therefore, they are best used alongside traditional methods rather than replacing them entirely.
Candida DNA Detection as a Fundamental Methodology
PCR-based methods detect Candida DNA from blood or body fluids within hours, offering faster diagnosis than blood culture. Limitations include false positives, inability to distinguish live from dead yeast, and no antifungal susceptibility data. Several commercial kits are available, including Magicplex, FungiPlex, and SeptiFast.
T2Candida® used T2 Magnetic Resonance to rapidly detect common Candida species with high specificity (>90%) and negative predictive value (98.8%), but sensitivity varied widely (7.7–68%) depending on infection type. It was fast and accurate but costly, could not determine drug susceptibility, and is no longer commercially available. Its best use is in patients with high risk of IC, often combined with other biomarkers for improved detection.
Reliance of Current Methods on Yeast Cell Wall Polysaccharide Detection
Mannan:
Mannan is a Candida cell wall polysaccharide detected by ELISA (e.g., Platelia™ Candida Ag Plus). Its rapid clearance and binding to antibodies reduce early detection sensitivity, reported as low as 14%.
(1,3)-β-D-glucan (BDG):
BDG is found in many fungi, not just Candida, but is sensitive for IC diagnosis. Commercial kits like Fungitell® and Wako GT® measure BDG in serum (>80 ng/L or >7 ng/L, respectively). It’s mainly used to rule out IC, but false positives are common from medical products, antibiotics, dialysis, or gut flora translocation.
Candida Score:
The Candida score predicts IC risk in ICU patients using four factors: TPN, surgery, multifocal colonization, and severe sepsis. A score >2.5 indicates high risk and early antifungal therapy is advised. Its accuracy varies by ICU population, so clinical context and additional risk factors should also be considered.
Challenges and Future Perspectives in the Diagnosis of Invasive Candidiasis
Advances in IC Diagnostics:
Over the past two decades, IC diagnostics have improved, yet challenges remain in achieving high sensitivity, specificity, and rapid species identification. Standardized, clinically validated molecular tests are crucial to improve early detection and guide targeted therapy, ultimately enhancing patient outcomes. Current literature emphasizes the need for fast, accurate, and reliable methods to reduce morbidity and mortality in IC.
Novel Detection Methods:
New approaches, including detection of fungal metabolites like D-arabinitol, show promise beyond traditional culture methods. Siderophores—small molecules involved in fungal iron metabolism—are emerging as potential IC biomarkers. Modified siderophores, labeled with radionuclides or fluorescent dyes, enable precise imaging and even targeted therapy. Research continues to identify Candida-specific siderophores, and integrating these novel tools with conventional methods may allow earlier diagnosis and timely antifungal treatment.
Reducing Time to Diagnosis:
A key challenge is minimizing delays between sample collection and lab processing. Many microbiology labs operate only during regular hours, delaying blood culture results by up to 15 hours, which can compromise timely antifungal therapy. Continuous lab operations, although costlier, allow rapid identification of Candida, susceptibility testing, and initiation of targeted treatment, reducing hospital stays, costs of ineffective therapy, and risk of resistant infections.
Measuring Quality of Care (EQUAL Candida Score):
The EQUAL Candida score evaluates adherence to international guidelines for candidemia management, including blood cultures, antifungal selection, CVC removal, echocardiography, and follow-up cultures. Scores range up to 22 (with CVC) and higher scores indicate better guideline adherence. Studies show higher EQUAL scores correlate with improved survival, though cutoff values and predictive accuracy vary. This tool helps monitor quality of care and identify areas for improvement in IC management.
Conclusion
The diagnosis and management of invasive candidiasis remain challenging due to its complex pathogenesis and limitations of traditional methods. Advances such as PCR-based tests, biomarker assays, and emerging tools like siderophores offer faster, more sensitive detection. Combining these novel approaches with conventional methods provides a comprehensive diagnostic strategy. Timely diagnosis, supported by continuous lab operations and rapid testing, is critical for initiating effective therapy, improving patient outcomes, and reducing healthcare costs.
Source: Slepčanová, H., Dobiáš, R., Langer, A.B.S. et al. Diagnostic Approaches to Invasive Candidiasis: Challenges and New Perspectives. Mycopathologia 191, 4 (2026). https://doi.org/10.1007/s11046-025-01035-4




