Introduction
(Article introduction authored by INCA Editorial Team)
Acinetobacter baumannii complex (ABC), particularly its carbapenem-resistant strains (CRAB), presents a significant challenge due to its high resistance to antibiotics and its role in severe infections such as pneumonia and septic shock.
The limited efficacy and toxicity of older treatments like colistin and polymyxins highlight the urgent need for new therapeutic options.
Recently, cefiderocol and sulbactam-durlobactam have emerged as promising alternatives for CRAB infections, and additional agents like BV-100, cefepime-zidebactam, zosurabalpin, and OMN6 are in advanced clinical trials.
Recently Approved Agents
Cefiderocol
An advanced generation cephalosporin, cefiderocol is a newer option for combating ABC infections (Table 1). Cefiderocol, a siderophore antibiotic, is approved in the US, Europe, and parts of Asia.
Effective against carbapenem-resistant ABC, P. aerugi nosa, and Enterobacterales spp., only 1.7% of ABC isolates showed non-susceptibility.
Observational studies suggest lower mortality with cefiderocol vs. colistin-based treatments, especially in CRAB pneumonia and COVID-19. Despite potential, IDSA and European guidelines reserve it for last-resort combination therapy. Further research is needed.
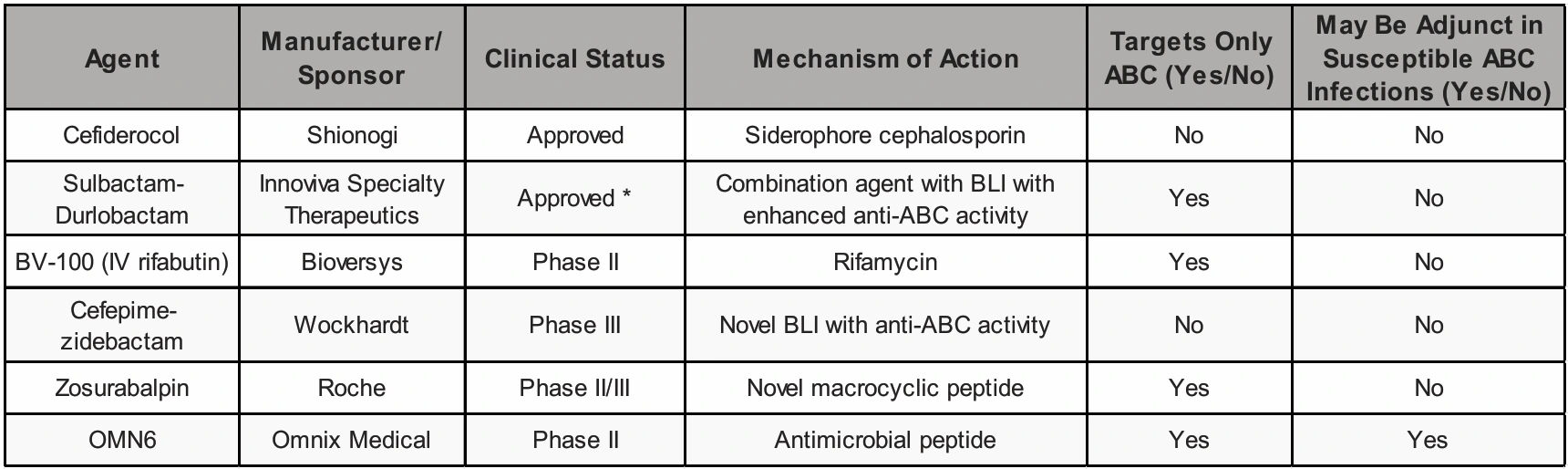
Table 1. Summary of Recently Approved Agents and Those Under Development.
Abbreviations: ABC—A. baumannii complex, BLI—beta-lactamase inhibitor, IV—intravenous. * Only in the United States.
Cefiderocol
Sulbactam-Durlobactam (SD) combines sulbactam, an older beta-lactamase inhibitor (BLI), with durlobactam, a novel BLI, recently approved in the US and under review in Asia but not yet compliant with EU regulations.
Sulbactam targets ABC infections, while durlobactam restores its activity against resistant strains. Clinical trials confirmed SD’s non-inferiority to colistin in CRAB infections, with lower nephrotoxicity and a trend toward improved survival. IDSA recommends SD with a carbapenem for CRAB, but real-world data are still limited.
Agents in Development
BV-100
BV-100 is an intravenous (IV) formulation of rifabutin, a rifamycin with strong activity against Acinetobacter baumannii (ABC). Unlike other rifamycins, BV-100 exhibits potent antibacterial effects with a minimal inhibitory concentration (MIC) of 0.0156 µg/mL.
Resistance to BV-100 is rare but can develop through efflux pumps or mutations in the rpoB gene. However, studies suggest that combining it with colistin may help mitigate resistance.
Phase I trials confirmed its safety and tolerability, and a Phase II trial is evaluating its effectiveness in treating severe Carbapenem-resistant A. baumannii (CRAB) infections, including nosocomial pneumonia and blood stream infections.
Cefepime-Zidebactam (WCK 5222)
Cefepime-Zidebactam (WCK 5222) is a combination antibiotic designed to combat multidrug-resistant
Gram-negative infections, particularly CRAB and ABC. It pairs cefepime, a fourth-generation cephalosporin, with zidebactam, a β-lactamase inhibitor (BLI) that targets PBP2. Unlike conventional BLIs, zidebactam enhances cefepime’s activity against A. baumannii, significantly improving its potency.
Zosurabalpin
Zosurabalpin is a novel macrocyclic peptide that targets ABC by disrupting lipopolysaccharide (LPS) transport via the LptB2FGC complex. It demonstrates exceptional potency against ABC, with an MIC90 of 1 mg/L, but has little activity against other Gram-negative bacteria.
OMN6
OMN6 is an antimicrobial peptide rather than a traditional antibiotic. It has shown strong activity against CRAB, including colistin-resistant strains, with a low MIC range of 4–8 mcg/mL.
Unlike many antibiotics, OMN6 remains effective in lung surfactant, making it a promising candidate for treating ABC pneumonia.
OMN6 represents a novel approach to fighting antibiotic-resistant infections with potentially fewer resistance challenges.
Conclusion
Two agents are now commercially approved for treating severe ABC and CRAB infections, each with unique
strengths and limitations.
The four agents in advanced development offer hope with novel mechanisms that may help curb resistance and improve survival rates, which currently reach 40%.
Until more evidence emerges, physicians must rely on infection control and antibiotic stewardship to manage ABC and CRAB infections effectively.
Source: Arshad, N.; Azzam, W.; Zilberberg, M.D.; Shorr, A.F. Acinetobacter baumannii Complex Infections: New Treatment Options in the Antibiotic Pipeline. Microorganisms 2025, 13, 356. https://doi.org/10.3390/microorganisms13020356