Introduction
Antimicrobial resistance (AMR) is a major global health threat. According to the CDC (2019), over 2.8 million antibiotic-resistant infections and 35,000 deaths occur annually in the U.S., rising to 3 million infections and 48,000 deaths when C. difficile cases are included. Similar findings are reported by the ECDC and WHO.
This review aims to guide clinicians on optimal antibiotic use in ICUs to improve outcomes and curb resistance. A MEDLINE search on antibiotics, resistance, stewardship, intensive care, and infection was used for this analysis.
Antibiotics are widely used in ICUs due to the high rate of severe infections, 43% to 60% of ICU patients globally, as reported in the EPIC III study. Resistant infections significantly increase hospital mortality. Overuse of antibiotics and altered drug pharmacokinetics in critically ill patients further contribute to resistance.
Given these challenges, this review emphasizes antibiotic stewardship and optimization in ICUs to enhance patient care while limiting the rise of resistant pathogens.
Initial empiric treatment and combination therapy
Given the high prevalence of sepsis and septic shock, ICU clinicians often need to start empiric antibiotics before identifying the pathogen. Once results are available, therapy is adjusted to definitive treatment. Recognizing infection early is vital, though diagnosis is often uncertain. Up to one-third of presumed sepsis cases may later prove non-infectious, leading to unnecessary antibiotic use.
Guidelines recommend early broad-spectrum antibiotics (within 1–3 hours) for septic shock, as each hour of delay lowers survival. However, rapid antibiotic use in all sepsis cases may harm uninfected patients, so a careful evaluation within 3–5 hours is advised.
Empiric choices should consider infection site, patient risk factors, and local resistance patterns. Overestimation of resistance often leads to overly broad therapy, worsening resistance and outcomes. Emerging machine learning tools aim to better predict resistance and support narrow-spectrum use where safe.
Combination therapy (e.g., beta-lactam + aminoglycoside) may be used only for severe infections with suspected resistant Gram-negative bacteria or specific mixed/toxin-producing infections.
In conclusion, timely and targeted antibiotics are crucial in sepsis. Broad coverage should be reserved for patients in septic shock or with high-risk infections, guided by local data, patient history, and stewardship principles. Future AI tools and rapid diagnostics may enhance accuracy and outcomes in antibiotic decisions.
Pharmacokinetic considerations
In critically ill or septic shock patients, altered antimicrobial pharmacokinetics (PK) due to organ dysfunction and supportive therapies (ventilation, ECMO, RRT, etc.) complicate dose optimization.
Research mainly focuses on beta-lactams and vancomycin. For beta-lactams, efficacy depends on maintaining drug levels above the MIC for sufficient time. Prolonged or continuous infusions improve target attainment, especially with high MIC pathogens, though large trials (MERCY, BLING III) found no mortality benefit over intermittent dosing.
Therapeutic drug monitoring (TDM) helps adjust doses to maintain adequate trough levels, but studies (TARGET, DOLPHIN) show no significant clinical improvement compared to standard dosing.
Despite inconclusive outcomes, optimizing therapy for patients with high MIC infections or augmented renal clearance remains essential. TDM should mainly be used to prevent drug toxicity, particularly with glycopeptides and aminoglycosides.
Antibiotic de-escalation strategies and duration of treatment
Antibiotic de-escalation (ADE) involves replacing broad-spectrum antibiotics with narrower ones, discontinuing unnecessary agents, or stopping antibiotics entirely when infection is no longer suspected. It is a key antimicrobial stewardship strategy that helps prevent adverse drug effects and limit antimicrobial resistance (AMR)—a global threat causing over 4 million deaths yearly, projected to rise by 70% by 2050.
Studies like DIANA and meta-analyses confirm that ADE is safe and effective, improving clinical outcomes without increasing mortality. Early de-escalation also reduces antibiotic toxicity and protects the gut microbiome, which prevents infections such as C. difficile.
Guidelines recommend daily assessment and ADE within 24 hours of culture results. With rapid diagnostic tests (RDTs)—like multiplex PCR, MALDI-TOF, or NGS—ADE can now be performed within hours instead of days.
Shorter antibiotic courses (3–7 days) have proven equally effective as longer regimens and reduce AMR risk, emphasizing the importance of timely ADE and optimal treatment duration guided by patient response and biomarkers like procalcitonin.

Figure 1 – Algorithm for antimicrobial strategy in the ICU balancing appropriate initial antibiotic treatment with the need to minimize resistance emergence by employing stewardship practices.
Antibiotics for infection prevention
Selective Digestive Decontamination (SDD) aims to prevent ICU-acquired infections by applying nonabsorbable and intravenous antibiotics to eliminate colonizing microbes at ICU admission. While widely used in the Netherlands, its adoption elsewhere is limited due to antimicrobial resistance (AMR) concerns.
The SuDDICU trial (2022) involving 6,000 ventilated patients showed no significant mortality reduction with SDD, though a meta-analysis of 25,000 patients suggested modest benefit. However, studies in high-AMR ICUs found no advantage of SDD against multidrug-resistant infections.
Inhaled antibiotics like colistin and amikacin have shown reduced ventilator-associated pneumonia (VAP) rates in some trials, but no improvement in mortality or ICU stay, possibly due to underdiagnosis from prior antibiotic exposure.
In patients with acute brain injury, the PROPHY-VAP trial showed that a single ceftriaxone dose reduced VAP incidence and improved ICU-free days, though culture-based definitions may underestimate infections.
Despite these findings, up to 50% of prophylactic antibiotic use in ICUs is inappropriate, often prolonged unnecessarily. Thus, careful assessment and antibiotic stewardship remain essential to balance infection prevention and resistance risks.
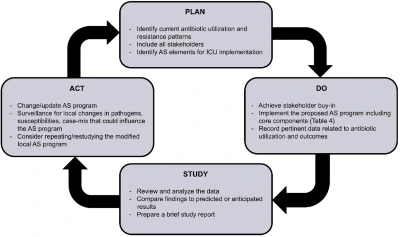
Figure 2 – A quality improvement implementation strategy for introducing and evaluating stewardship practices in the ICU.
Conclusions
Antibiotics are widely used in ICUs, impacting both current and future patients due to resistance development. To preserve their effectiveness, ICU teams should routinely adopt antibiotic optimization strategies. A multifaceted approach ensures better patient outcomes while minimizing resistance and safeguarding antibiotics for long-term use.
Source: Micek, S.T., Vazquez Guillamet, M.C., Reynolds, D. et al. Optimal antibiotic use in the intensive care unit. Crit Care 29, 434 (2025).
https://doi.org/10.1186/s13054-025-05653-8