Introduction
Sepsis-Associated Acute Kidney Injury (SA-AKI) is a critical condition with high morbidity and mortality in intensive care unit patients.
Extracorporeal blood purification therapies have been developed for managing sepsis and SA-AKI, but questions regarding their timing, efficacy, and indications persist.
Key unresolved issues include determining when to start renal replacement therapy in septic vs. non-septic acute kidney injury, defining the optimal dialysis dose for SA-AKI, and understanding the rationale for using blood purification therapies in septic patients without acute kidney injury.
Novel therapies, such as those using adsorption devices, have sparked interest in clearing specific mediators implicated in organ dysfunction and removing antibiotics.
The joint commission of the Italian Society of Anesthesiology and Critical Care (SIAARTI) and the Italian Society of Nephrology (SIN) has addressed these issues, proposed clinical practice recommendations, and established a framework for future research in this field.
Section 1. Extracorporeal therapies for sepsis in the absence of AKI
Q1: Is there a rationale for using EBPT for sepsis in the absence of AKI?
Consensus statements:
Despite significant advances of EBPT and the introduction of novel approaches targeting different phases of the immune response to sepsis, no evidence supports the regular use of such therapies in addition to the standard of care.
Extracorporeal blood purification therapies should therefore be personalized according to the patients’ specific conditions and needs, mainly in the setting of controlled clinical trials aimed at evaluating the efficacy and the optimal timing of EBPT.
Study registries may be helpful to clarify the clinical results and the rationale for using EBPT in clinical practice.
Rationale
Despite advances in sepsis diagnosis and management, it remains a global health concern with rising incidence and mortality.
Antibiotic resistance and the absence of specific therapies pose challenges.
Extracorporeal blood purification therapies (EBPTs) have been suggested for sepsis treatment, targeting the dysregulated host response and organ dysfunction.
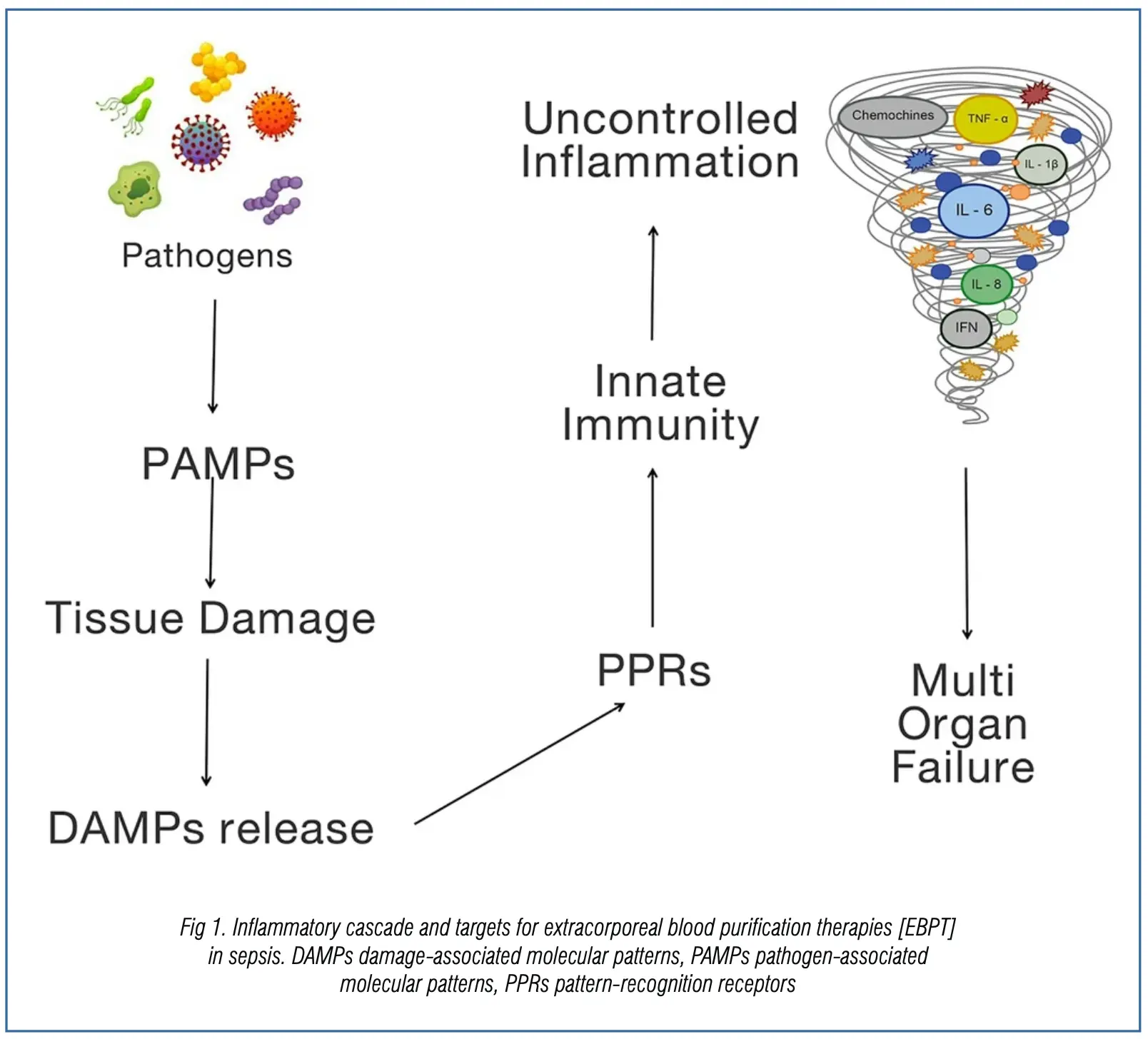
These therapies aim to remove specific triggers, induce immunomodulation, and potentially contribute to organ protection (Fig. 1).
Various EBPTs act by removing pathogen-associated molecular patterns (PAMPs) and cytokines.
However, their use is controversial due to potential adverse effects, limiting widespread adoption.
Personalized use based on patients’ needs, risks, and benefits is recommended, with emphasis on research through randomized controlled trials and study registries.
Section 2. Kidney support in sepsis-associated AKI
Q2: Should the indications for Renal Replacement Therapies be different in patients with septic versus non-septic AKI?
Q3: Should the timing of RRT be different in patients with septic versus non-septic AKI?
Consensus statements:
The available RRT for SA-AKI can provide fluid and solute control and blood purification through diffusion, convection, and adsorption as described for other forms of ischemic and nephrotoxic AKI.
Indications for initiating and discontinuing RRT do not differ between SA-AKI and other types of AKI.
Depending on local resources and clinical practice, the combination of kidney and immunomodulatory support in SA-AKI patients could be considered.
Future evaluation of specific clinical and biological criteria (e.g. plasma concentrations of measurable PAMPs/DAMPs) could be useful to guide the selection of patients for RCTs (not graded).
Rationale
Sepsis-associated acute kidney injury (SA-AKI) worsens outcomes, especially for those requiring renal replacement therapy (RRT).
Recent studies emphasize differences in SA-AKI mechanisms, involving toxic and immunologic factors. Fluid overload, a key RRT indication, is associated with organ dysfunction and increased mortality.
Early vs. late RRT initiation trials show controversial results, with personalized approaches recommended. Biomarkers, like NGAL/cystatin C and TIMP-2/IGFBP-7, aid RRT initiation prediction, though routine use lacks evidence.
Extracorporeal blood purification therapies targeting pathogen-related mediators are studied, but guidelines currently don’t endorse them. Further research should assess EBPTs’ effects on real-life ICU outcomes, considering various clinical endpoints and long-term organ function.
Q4: What is the optimal dialysis dose in the critically ill patient with SA-AKI?
Consensus statements:
Higher RRT doses [prescribed effluent fluid rate > 35–40 mL/kg/h] do not seem to favorably impact outcomes in critically ill patients with SA-AKI.
The delivered RRT dose represents a dynamic quality indicator that should be systematically monitored to individualize the treatment according to specific solute and volume control goals.
Based on clinical practice and experience of the center, consider higher RRT dose if needed to meet specific targets in selected clinical situations (not graded).
Rationale
The optimal dose of renal replacement therapy (RRT) in acute kidney injury (AKI) remains debated. Studies on different RRT modalities report controversial results.
The “Vicenza study” showed greater survival with a higher prescribed dialytic dose, while other studies found no significant mortality differences.
Larger trials like the ATN and RENAL studies failed to demonstrate improved outcomes with more intensive RRT. A Cochrane review and meta-analysis did not show improved mortality or kidney recovery with higher RRT doses, except in post-surgical AKI.
KDIGO AKI guidelines recommend an average effluent dose of 20–25 mL/kg/h. Delivered doses should be monitored, considering interruptions and downtime factors.
No proven strategies compensate for decreased delivered doses, emphasizing the importance of personalized RRT approaches. In summary, there is no evidence supporting higher RRT doses (>35–40 mL/kg/h) over standard doses (25–30 mL/kg/h) in critically ill AKI patients, even in sepsis-associated AKI.
Section 3. Immunomodulatory support in SA-AKI
Q5: Which EBPTs aimed at removing PAMPs and/or DAMPs could affect organ dysfunction?
Consensus statements:
The efficacy of EBPTs in terms of septic shock resolution found in observational clinical studies has not been proven in RCTs.
Although none of the EBPTs has shown a benefit in terms of mortality when prescribed as adjuvant therapy of sepsis, some positive results on organ dysfunction have occasionally been observed.
Possible strategies based on clinical and humoral biomarkers are needed to predict which patients might benefit from a personalized medicine approach.
Rationale
Evolving from the understanding of cytokine-mediated immune responses, extracorporeal blood purification therapies (EBPTs) aim to mitigate the life-threatening “cytokine storm.” Techniques include high-volume hemofiltration, high cut-off membranes, and adsorption.
However, larger randomized controlled trials (RCTs) have not demonstrated clear benefits, especially in terms of mortality. In septic shock, a cytokine absorption device showed reduced IL-6 and vasopressor requirements, but increased ICU mortality.
Similar outcomes were observed in an RCT for COVID-19 patients on ECMO using a cytokine
adsorber device. The COMPACT-2 study for septic patients using Coupled Plasma Filtration Adsorption was prematurely stopped due to increased mortality in the treated group.
Presently, routine use of EBPTs in sepsis-associated acute kidney injury is not supported by current evidence. Further research is needed to determine clinical efficacy and identify potential enrichment strategies based on bedside biomarkers.
Q6: Could the removal of endotoxins by specific EBPTs affect organ dysfunction?
Consensus statements:
Septic shock patients with a MODS score > 9 and endotoxin activity assay (EAA) level ranging from 0.6 to 0.9 are those who may mostly benefit from polymyxin-B hemoperfusion.
Although the results on mortality are still unclear, endotoxin removal has been shown to improve organ dysfunction as assessed by the Sequential Organ Failure Assessment (SOFA) score.
The decision to initiate PMX-B alone or in combination with RRT still remains based on local clinical practice and individual opinion of the physicians.
This specific treatment should be performed by a well-trained multidisciplinary team.
Rationale
Endotoxin, a harmful pathogen-associated molecular pattern (PAMP) in septic shock, has led to the exploration of extracorporeal endotoxin neutralization therapies.
Polymyxin B-immobilized polystyrene-derived fiber hemoperfusion (PMX-B) aims for direct endotoxin removal but lacks definitive evidence supporting its efficacy in septic shock.
Clinical trials yielded conflicting results, with recent meta-analyses presenting controversial data on mortality reduction.
EUPHRATES RCT suggested a survival benefit in patients with septic shock and endotoxin activity assay (EAA) levels between 0.6 and 0.89. A proposed golden hour strategy recommends targeted PMX-B use based on endotoxic shock diagnosis, incorporating regular EAA evaluations, source control, cultures, and antibiotics.
However, insufficient evidence and conflicting guidelines currently exist, leaving decisions on PMX-B initiation, modality, timing, and duration to local clinical practices and individual physician opinions.
Section 4. Antibiotic removal and EBPT in SA-AKI
Q7: How can EBPT affect antibiotic removal in patients with SA-AKI?
Consensus statements:
AKI and EBPT can both affect volume of distribution and antibiotic clearance, often resulting in subtherapeutic plasma levels, lower efficacy and increased mortality rate.
The modality of dialysis, the reinfusion site and the properties of membranes/devicesff could significantly interfere with pharmacokinetic/pharmacodynamic parameters associated with drug efficacy by influencing antibiotic removal.
To optimize antibiotic dosage and maximize effectiveness, it is important to individualize the antimicrobial therapy based on the patient and the EBPT used.
Rationale
Antibiotic dosing in SA-AKI is complex, involving factors such as clinical context, infection source, and AKI stage. No validated guidelines exist for adjusting antibiotic doses in these patients.
Pharmacokinetic and pharmacodynamic optimization, considering fluid balance changes during sepsis and AKI, is recommended. SA-AKI and extracorporeal blood purification therapies (EBPT) impact antibiotic clearance, often leading to subtherapeutic levels.
Antibiotic dosing considerations should include molecular features, standard RRT/EBPT modalities, and filter membrane types. EBPT, involving convective, diffusive, or mixed techniques, affects antibiotic clearance differently.
High-intensity RRT may require higher antibiotic doses, while the choice of dialyzer and adsorptive devices further complicates dose determination.
Individualized antibiotic therapy, considering patient characteristics and RRT/EBPT methods, is crucial, demanding a deep understanding of EBPT modalities and their effects on drug clearance, along with the influence of sepsis on antibiotic pharmacokinetics/pharmacodynamics.
The lack of standardization in EBPT and RRT practices leads to variable recommendations and challenges in managing SA-AKI patients.
Conclusions
Sepsis-associated acute kidney injury (SA-AKI) is defined as AKI occurring within 7 days of sepsis development, diagnosed according to KDIGO and Sepsis 3 criteria.
Identification of distinct SA-AKI endotypes, as defined by the 28th Acute Disease Quality Initiative workgroup, offers crucial prognostic insights. Indications for starting renal replacement therapy (RRT) in SA-AKI are similar to other renal dysfunctions.
Extracorporeal blood purification techniques (EBPT) may be considered for immunomodulatory support in SA-AKI patients meeting specific clinical and biological criteria based on the measurement of detrimental molecules.
Source: De Rosa, S., Marengo, M., Fiorentino, M. et al. Extracorporeal blood purification therapies for sepsis-associated acute kidney injury in critically ill patients: expert opinion from the SIAARTI-SIN joint commission. J Nephrol 36, 1731–1742 (2023). https://doi.org/10.1007/s40620-023-01637-5




